
Detecting total and phospho-ser129 α-synuclein in preclinical models
The Role of α-Synuclein in Parkinson’s Disease:
Synucleinopathies are a subtype of neurodegenerative disorders characterized by excessive accumulation and aggregation of α-Synuclein—a presynaptic protein involved in synaptic regulation and neurotransmitter release (1). This accumulation of α-Synuclein disrupts cellular homeostasis within the brain, triggering neuroinflammation and widespread neuronal loss. This inevitably leads to irreversible cognitive and motor impairment. Parkinson’s disease (PD) is one of the most prevalent synucleinopathies, marked by the loss of dopaminergic neurons and the aggregation of α-Synuclein. The precise mechanism driving α-Synuclein phosphorylation at serine 129 (pS129) and its aggregation of this protein remain unclear yet are integral components to the pathology of PD and other synucleinopathies, including dementia with Lewy bodies (DLB) (2).
Current Challenges with Detecting α-Synuclein:
While the pathological role of α-Synuclein is well-recognized, existing assays fall short in reliably detecting α-Synuclein at low levels, particularly in mouse models crucial for progressing PD and DLB research. Advances in immunoassays offer promising tools for detecting and quantifying α-Synuclein, particularly in its aggregated and phosphorylated forms.
In the article published by Trist et al. (3), the authors introduce an innovative approach using reformulated AlphaLISA SureFire Ultra (ALSU) assays to measure total and phosphorylated (pS129) α-synuclein with remarkable sensitivity and specificity in a no-wash, single-plate format. They further demonstrate the utility of AlphaLISA technology for advancing synucleinopathy research by enabling high-throughput, cross-species quantification of α-synuclein in both mouse and human brain tissue and cellular models.
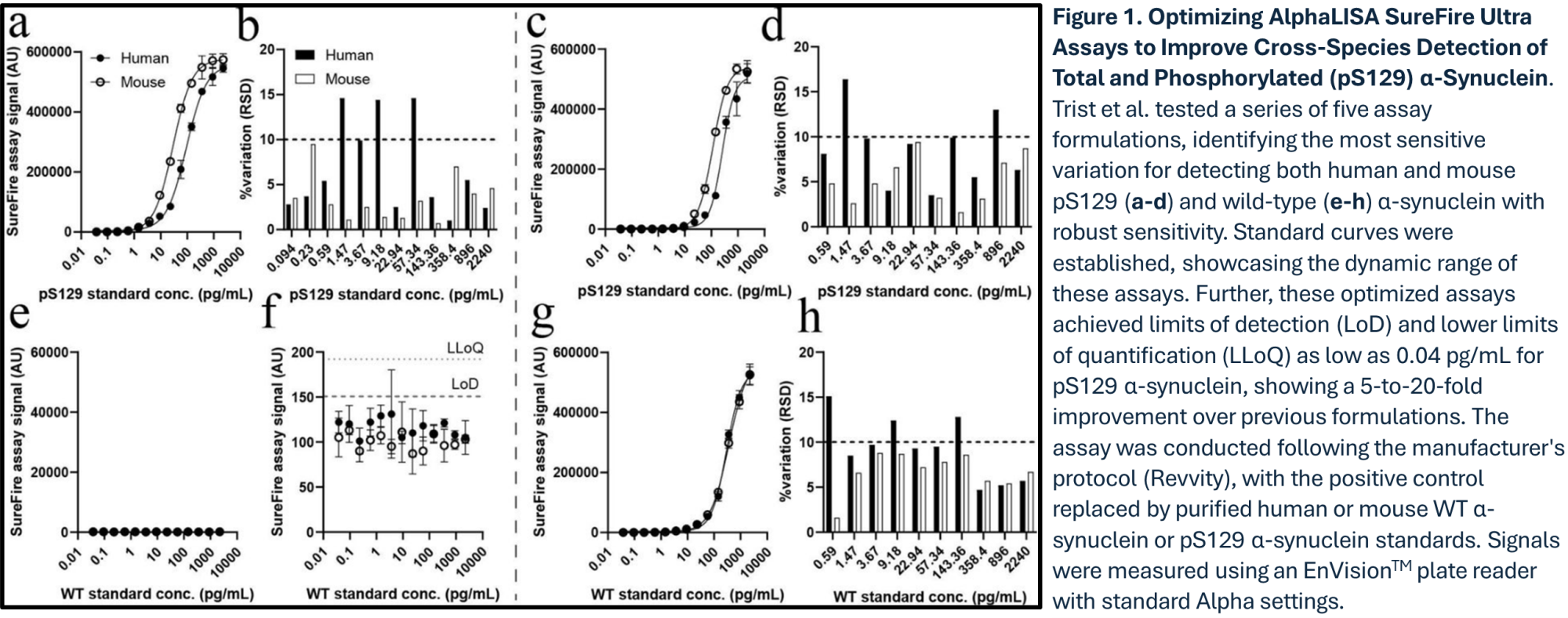
Validation of Reformulated AlphaLISA SureFire Ultra Assays in Complex Matrices:
Following the optimization process for identifying the most sensitive assay formulation as outlined in Figure 1, the authors then further validated the reformulated AlphaLISA SureFire Ultra assays in complex biological samples including mouse brain tissue and HEK293 cells (Figure 2). Both pS129 and total α-synuclein assays retained high sensitivity and selectivity, showing minimal non-specific signals in knock-out (KO) models. Background signals remained well-controlled, ensuring reliable differentiation between mouse wild-type and KO extracts. The specificity achieved in these complex matrices underscores the assay's robustness in diverse samples, making the platform instrumental for advancing preclinical research on synucleinopathies.
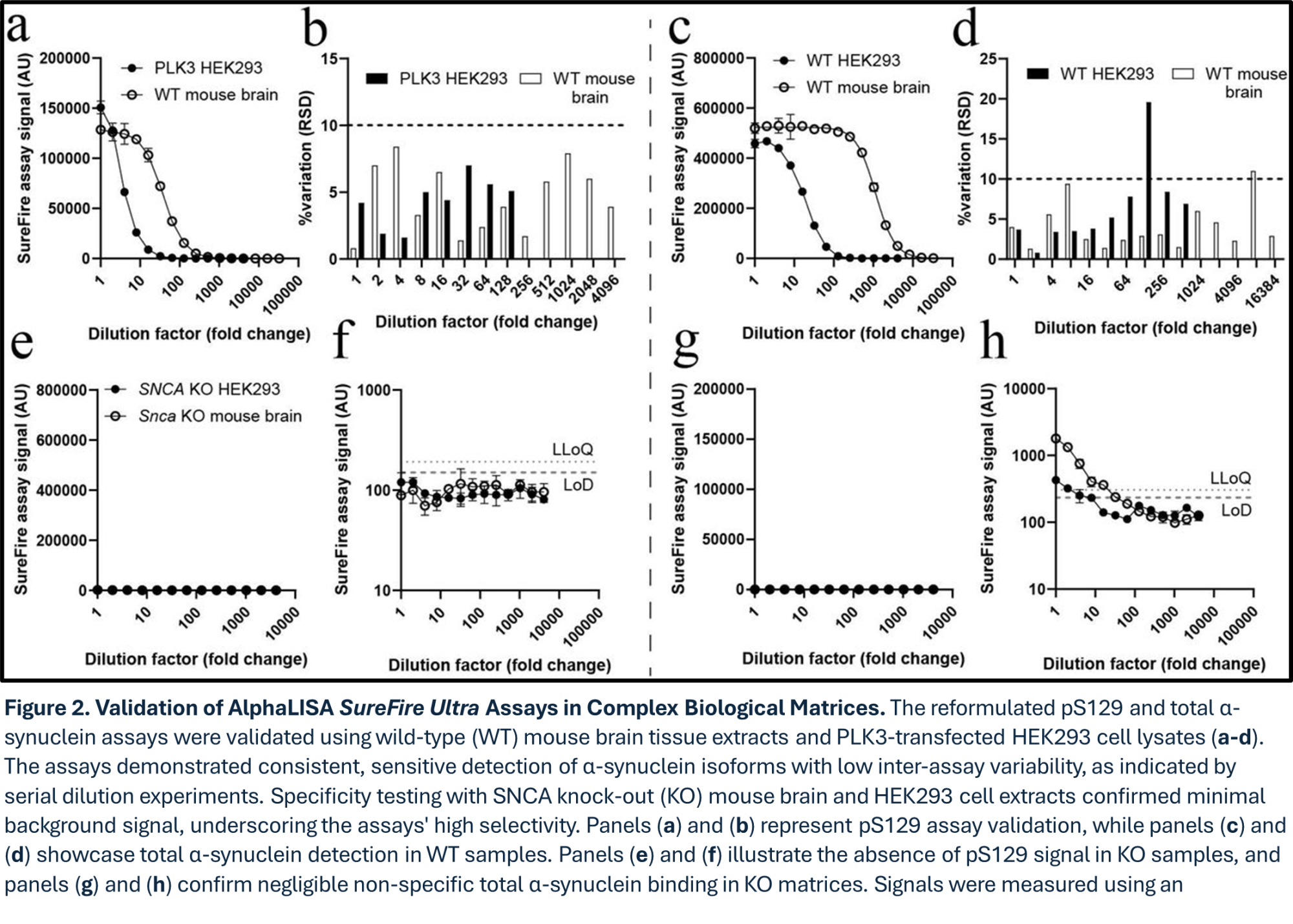
Evaluation of Matrix Effects on ALSU Assay Performance:
Trist et al. further assessed the impact of matrix effects on the reformulated AlphaLISA SureFire Ultra assays as depicted in Figure 3 using WT and SNCA-KO mouse brain extracts. Parallelism experiments revealed minor shifts in assay responses at 10-fold dilutions, which were resolved at 100-fold dilutions. Spike-in experiments further validated the accuracy of the reformulated assays. The pS129 α-synuclein assay demonstrated recovery rates between 80% and 92%, while the total α-synuclein assay exhibited recovery rates ranging from 79% to 94%. These results confirmed the robustness of the AlphaLISA technology, enabling accurate and reproducible quantification of α-synuclein across various complex biological samples, including brain tissue extracts.

Exploring the Relationship Between α-Synuclein Aggregation and S129 Phosphorylation
As a continuation from the experiments evaluating matrix effects on the ALSU assays' performance in complex matrices, the authors next investigated the relationship between α-synuclein phosphorylation and aggregation across various brain regions in pre-formed fibril (PFF)-inoculated mice (Figure 4). To explore this, BioLegend’s LEGEND MAX™ α-Synuclein Aggregate ELISA kit was used to quantify the abundance of aggregated α-synuclein in both PBS-soluble and Triton X-100-soluble brain tissue fractions from PFF-inoculated and sham-treated mice. In brain regions exhibiting high pS129 α-synuclein pathology, up to 44.6 ng of aggregated α-synuclein per mg of total protein was detected, with the majority of aggregates found in membrane-bound fractions (53.4–66.2%). In contrast, brain regions with moderate pathology showed lower levels of aggregation (2.7–4.6 ng/mg total protein), with most aggregates localized in membrane-bound fractions (78.3–100%). This experiment allowed for a detailed examination of how α-synuclein aggregation correlates with phosphorylation levels across various brain regions with differing levels of synucleinopathy.
By combining the reformulated AlphaLISA SureFire Ultra assays for detecting total and phosphorylated S129 α-synuclein levels with the BioLegend α-synuclein aggregate ELISA assay, the authors were able to provide novel insights into α-synuclein biology within established synucleinopathy mouse models. This integration enabled for the detection of multiple targets to provide a comprehensive understanding of α-synuclein dynamics in the brain under both physiological and pathological conditions.
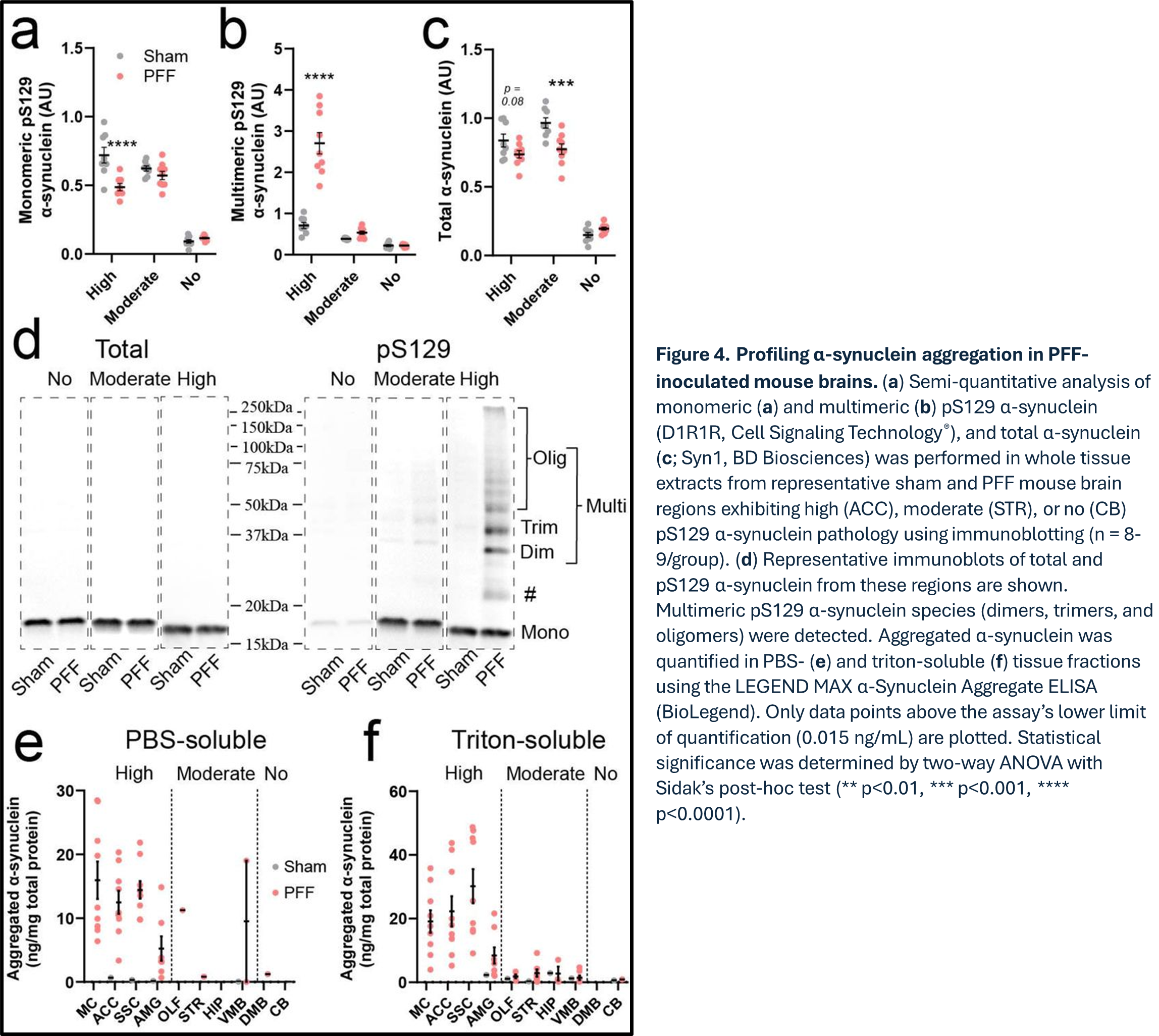
Other Notable Findings by Trist et al.:
- pS129 Levels and Pathological Burden: Regions with significant pathological burden in PFF-inoculated mice showed higher pS129 levels, particularly in cortical and limbic areas.
- Correlation with Total α-Synuclein: A consistent correlation was found between total α-synuclein concentration and regions susceptible to synucleinopathy, suggesting that pre-existing α-synuclein levels predispose regions to pathology.
- Regional α-Synuclein Compartmentalization: PFF-inoculated mice exhibited regional compartmentalization of α-synuclein, with membrane-bound forms contributing significantly to synucleinopathy pathology.
Conclusion:
The reformulated AlphaLISA SureFire Ultra assays (Revvity) and LEGEND MAX α-Synuclein Aggregate ELISA assay (BioLegend) presented by Trist et al. mark a significant advancement in detecting both mouse and human α-synuclein, enabling the direct study of total levels, phosphorylation of pS129 and protein aggregation in preclinical models. Further, the authors highlight the assays’ compatibility with complex matrices without sacrificing sensitivity. These assays offer improved insights into α-synuclein pathology, facilitating future research into targeted therapies for Parkinson’s disease and related disorders.
About AlphaLISA Technology:
AlphaLISA (Amplified Luminescent Proximity Homogenous Assay) is a bead-based, no-wash homogeneous assay platform, enabling the detection of analytes and a dynamic range of biological interactions, including protein-protein, protein-peptide, and protein-small molecule interactions. This technology utilizes streptavidin-coated Alpha Donor beads and AlphaLISA Acceptor beads. Upon excitation, a singlet oxygen molecule is generated in the Donor bead that triggers a series of chemical reactions in the Acceptor bead, resulting in the emission of light at 615 nm, which is directly proportional to the amount of analyte or binding events within the sample. Alpha technology allows for the direct detection of molecules of interest in a homogeneous format, eliminating the need for time-consuming wash steps.
AlphaLISA® SureFire® Ultra™ Technology:
The AlphaLISA SureFire Ultra kits from Revvity are particularly suited for this type of research. These kits offer numerous advantages, including exceptional sensitivity, a wide dynamic range, and the ability to detect interactions involving low-affinity binders. These features make AlphaLISA an ideal platform for a broad array of applications, from basic research to drug discovery and development. For example, the reformulated AlphaLISA SureFire Ultra (Cat. #ALSU-TASYN-B) and (Cat. #ALSU-PASYN-B) kits were essential for quantifying pS129 and total α-synuclein in this study, underscoring their utility in synucleinopathy research.

- AlphaLISA SureFire Ultra Human and Mouse Total α-Synuclein Kit
- AlphaLISA SureFire Ultra Human and Mouse Phospho-α-Synuclein (Ser192) Kit
- LEGEND MAX™ Human α-Synuclein Aggregate ELISA Kit
- AlphaLISA Technology Overview: Revvity - Alpha Assays
- AlphaLISA® SureFire® Ultra™ Product List and Optimization Guide: AlphaLISA SureFire Ultra: Unlock Precision in Phosphorylation Analysis
Link to Revvity’s Blog: α-Synuclein Blog
References:
1.) Gómez-Benito M, Granado N, García-Sanz P, Michel A, Dumoulin M, Moratalla R. Modeling Parkinson's Disease with the Alpha-Synuclein Protein. Front Pharmacol. 2020 Apr 23;11:356. doi: 10.3389/fphar.2020.00356. PMID: 32390826; PMCID: PMC7191035.
2.) Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002 Feb;4(2):160-4. doi: 10.1038/ncb748. PMID: 11813001.
3.) Trist, B.G., Wright, C.J., Rangel, A. et al. Novel tools to quantify total, phospho-Ser129 and aggregated alpha-synuclein in the mouse brain. npj Parkinsons Dis. 10, 217 (2024). https://doi.org/10.1038/s41531-024-00830-y
Written by Juliette Seremak, PhD
Commercial Technical Writer (Drug Discovery Reagents), Revvity
Images



